Overview
The brain is made up by many different cells, including neurons, which constitute the electric circuitry responsible for brain functions, and astrocytes, which provide the structure and support for neurons to work properly. Astrocytomas are tumors which originates from astrocytes, and, in adult individuals, they are the most common brain tumors. In the US, about 15,000 new astrocytomas are diagnosed every year. Males are slightly more affected than females, with a ratio of 1.3/1.
A Neurosurgeon Explains: Astrocytoma Tumors
Vikram C. Prabhu, MD, FAANS
Read the Full Transcript
Tumor Grading and its Meaning
According to the World Health Organization (WHO) classifications of brain tumors, astrocytomas range from grade 1 (most benign) to grade 4 (most malignant). This grading, which is made by analyzing the tumor cells under the microscope is based on the following features: 1) how abnormal the cells look like (atypia); 2) how much they grow (mitosis); 3) presence of newly made blood vessels within the tumor (vascular proliferation). This is further integrated by the analysis of tumor’s genetic features, i.e. the DNA analysis of the tumor cells. Generally, with the exception of grade 1 tumors, which are most common in the pediatric population, most astrocytomas affect patients older than 40. Also, the older the patient, the higher the chances of the astrocytoma to be of higher grade.
Grade 1
Pilocytic Astrocytoma is a well circumscribed tumor and grows slowly. Most common in the cerebellum, i.e. the part of the brain located in the back of the head, just above the neck. It does not invade into the surrounding brain, thus when resected completely it is considered cured, and it does not require either chemotherapy or radiotherapy.
Pleomorphic Xantoastrocytoma most frequently originates in the temporal lobes and is commonly associated with seizures. Its cells can have many different shapes (pleomorphic), but usually do not show evidence of proliferation. Surgery is usually curative.
Subependymal Giant Cell Astrocytoma (SEGA) is most common in the younger population, usually in association with a familiar syndrome called tuberous sclerosis. It characteristically grows inside the ventricles, which are fluid-filled spaces deep into the brain, and can often block the normal outflow of this fluid, thus causing hydrocephalus. Surgical resection is usually curative.
Grade 2
Diffuse Astrocytoma is an invasive tumor, so there is no clear separation from the surrounding brain, and surgery itself might not be enough for its cure (this depends on several other factors described below). The tissue appearance is only moderately different from a normal brain, but cells appear abnormal under the microscope and slightly increased in number.
Grade 3
Anaplastic Astrocytoma is considered a more malignant evolution of a previously lower grade astrocytoma, which has acquired more aggressive features, including a higher pace of growth and more invasion into the brain. Histologically, it displays a higher degree of cellular abnormalities, and evidence of cell proliferation (mitoses), in comparison to grade 2 tumors. Surgery is never considered curative for these tumors, and needs to be followed by radiation and almost always chemotherapy.
Grade 4
Glioblastoma (GBM) is the most malignant, aggressive and common (60%) form of astrocytomas. Histologically, it is characterized by very abnormal-appearing cells, proliferation, areas of dead tissue and formation of new vessels. GBM can present either as a malignant progression from a previously existing lower grade astrocytoma (usually in 10% of cases) or originate directly as a grade 4 tumor (90% of cases). The former scenario is most common in younger patients, while the latter is most common after age 60. Regardless of its presentation, this tumor is a highly aggressive cancer, with pronounced brain invasion and destruction and very fast progression.
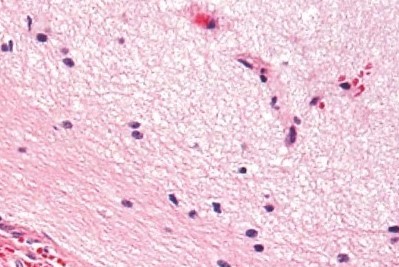
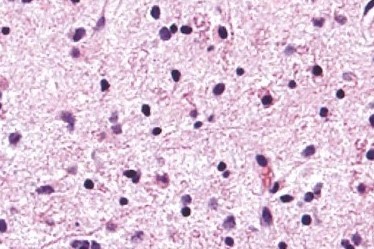
Diffuse astrocytoma
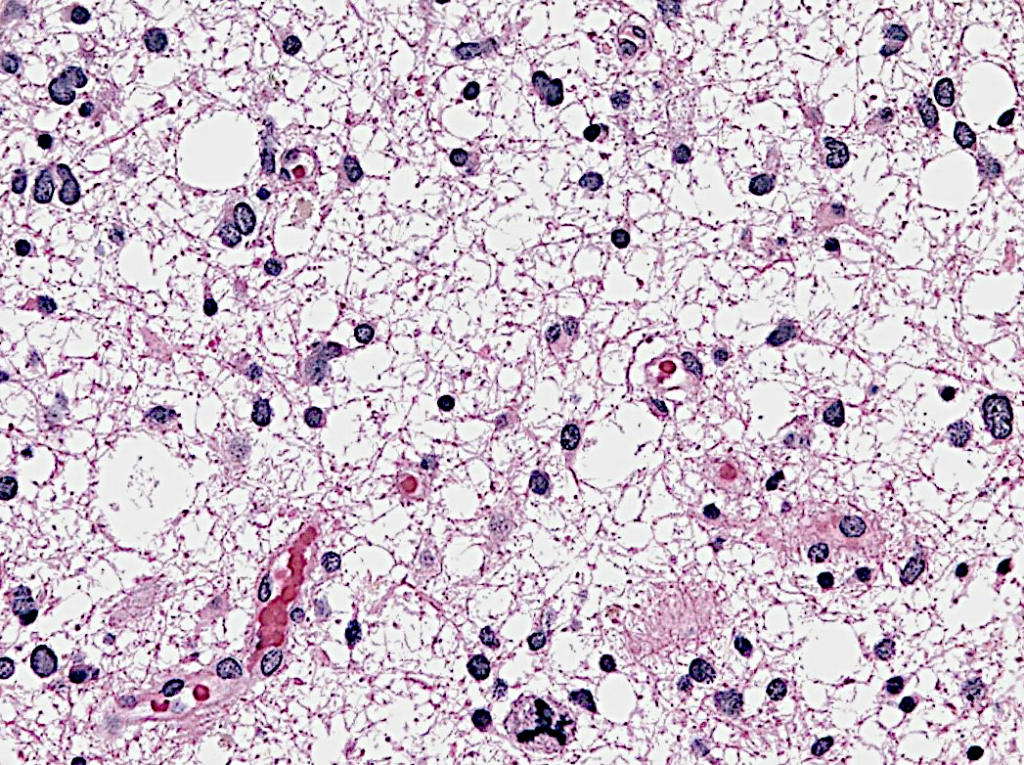
Anaplastic astrocytoma
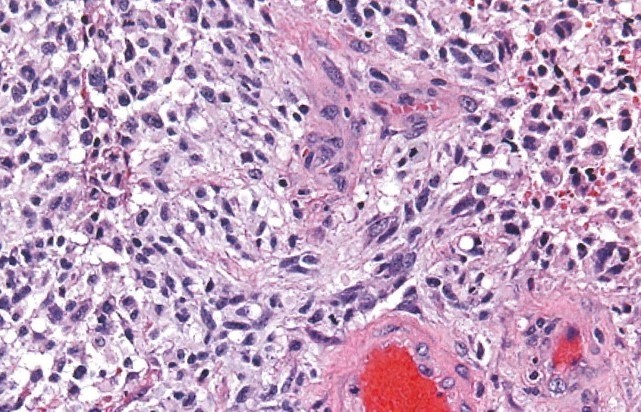
Glioblastoma
Modified from: Hannes Vogel’s Nervous system, Cambridge University Press, Lawrence Weiss, ed. 2009
Important Biological Features
Over the past 15 years, there have been important strides in understanding this disease, mainly derived from significant advances in the ability to study its genetic and biologic underpinnings
- IDH1 mutation is a fundamental feature that characterizes a subgroup of astrocytomas with mutation of a gene, called isocitrate dehydrogenase 1 (IDH1). This gene is involved in providing energy to the cells. Its mutation results in production of a chemical called 2-HG, which, over time, builds up inside the normal astrocytes and set them astray, causing astrocytomas. More than just another laboratory finding, this mutation is crucial because it characterizes almost 100% of low grade astrocytomas and is associated with significantly
- MGMT silencing has to do with the methylguanine-DNA methyltransferase (MGMT) enzyme involved in repairing DNA after it has been damaged by chemotherapics, thus protects the tumor against the action of these drugs. Certain astrocytomas have this enzyme turned off, and, as a result, they respond significantly better to chemotherapy with TMZ.
- Immunologic escape means that tumors escape the patrolling immune system, which normally destroys anything that is recognized as “abnormal”. Astrocytomas are among the tumors with the most developed ability to escape this surveillance. They do this by activating multiple genes that can turn the immune system off. There is consensus that counteracting this immune escape will result in important therapeutic benefits.
Risk Factors
Astrocytomas are, for the vast majority, sporadic tumors, meaning that they happen by chance, or at least, it is not yet known why these occur. There are only two situations with proven evidence to cause the tumor:
- Hereditary syndromes (i.e. caused by inherited DNA mutations):
- Li-Fraumeni: due to mutation in tumor suppressor gene p53 and characterized by young onset of multiple tumors, including breast cancer, bone cancers, leukemias and astrocytomas.
- Turcot: due to mutations in several tumor suppressor genes, including APC and MMR and characterized by early onset of colon cancer and astrocytomas.
- Neurofibromatosis 1: due to mutation of tumor suppressor NF1, responsible for early onset of astrocytomas, peripheral nerve tumors, skin freckling and light-brown patches in the skin.
- Tuberous sclerosis: is a rare genetic disorder associated with mental retardation and early onset of subependymal giant cell astrocytoma (SEGA).
- Environmental:
- Ionizing radiations: exposure to ionizing radiations has been associated to delayed onset of astrocytomas. Individuals at particular risk are those exposed to therapeutic radiotherapy to the head and neck region during childhood (i.e. for treatment of leukemias or other brain tumors). The interval between exposure to radiation and astrocytoma onset can be as long as 20-30 years.
- Warfare chemicals: There is a yet unproven suspicion that exposure to Agent Orange during the Vietnam War might be responsible for delayed onset of astrocytomas in veterans.
- Cellular phones: despite some suspicions, particularly related to heavy use, there is no data supporting a causative risk for astrocytomas.
SYMPTOMS + TYPES
The clinical presentation of astrocytomas depends much more on their location within the brain, rather than their biologic characteristics. There are regions of the brain that can accommodate very large tumors before they become symptomatic (for example, the regions in the forehead), while there are other locations where even a small tumor can cause problems early on, like limb weakness or difficulty with speech or vision.
Generally, low grade astrocytomas tend to be of bigger size before they become symptomatic, as compared to more aggressive, higher grade astrocytomas. This is because lower grade tumors tend to displace the brain rather than destroying it, and also because they are associated with less brain swelling than malignant ones.
Common symptoms of astrocytomas are the following:
- Persistent headaches
- Headaches which are worse in the morning or cause awakening from sleep ( a sign of increased intracranial pressure)
- Double or blurred vision
- Speech problems
- Decreased cognitive abilities
- Grasp or limb weakness
- New seizures
TESTING + DIAGNOSIS
Diagnostic Imaging
Conventional MRI: Magnetic resonance imaging (MRI) is the most important imaging study for astrocytomas. Usually, images are acquired both before and after the administration of IV contrast. As a rule of thumb, if the tumor picks up the contrast (i.e. becomes bright on images), it is an indication of a higher grade astrocytoma.
Other imaging sequences provides clues as to tumor cellularity, brain swelling and brain infiltration.
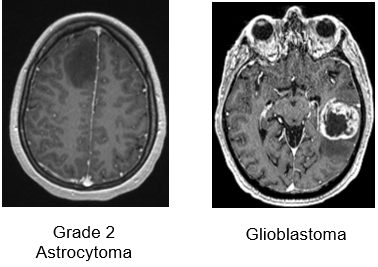
Axial T1-weighted MRIs after IV gadolinium administration:
Low grade tumors usually do not take contrast (left panel), while grade 4 tumors display strong contrast enhancement and frequent central necrosis (right panel)
MRI spectroscopy (MRS): It is an imaging tool, based on MRI, which provides information on the chemical composition of the tumor, and works based on the fact that certain chemicals are abundant in the normal brain (for example, NAA), while others are abundant in tumors (for example, choline). The output of this imaging modality is a diagram showing the amount of each chemical in the area of the brain under analysis: If the amount of NAA is more than choline, that suggests a normal brain (see below). The opposite raises suspicion of a tumor. This technique can be considered as a non-invasive tissue sampling, although it is not as accurate or definitive as a standard biopsy.
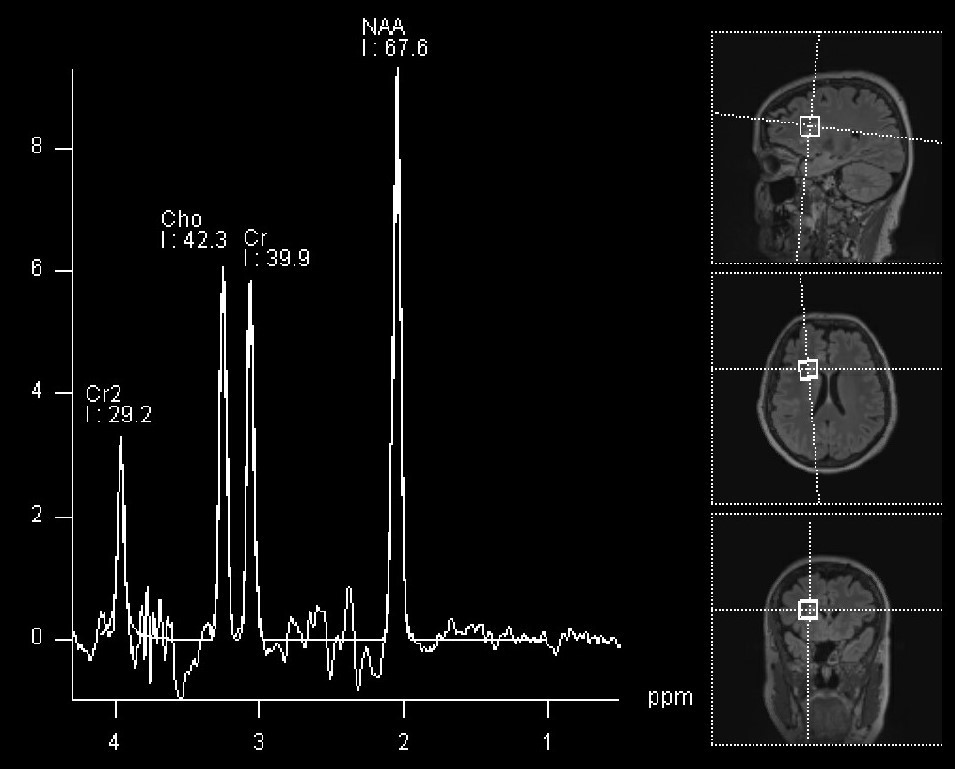
MRI spectroscopy of normal brain (sampled voxels are represented on the right side of the panel).
The NAA peak is the most prominent
Functional MRI (fMRI): fMRI is a useful technique to visualize in real time which parts of the brain become activated when the patient is asked to perform a certain task (for example talking, or moving one arm or leg). This is fundamental to define the regions of the brain which, if damaged, would cause problems to the patient. Activated brain is shown as a yellow/red signal (see figure below) superimposed to an otherwise standard MRI. For tumors that are localized in the proximity of critical areas (speech centers, motor cortex or visual cortex) fMRI provide an important adjunct, particularly in regards to surgical planning.
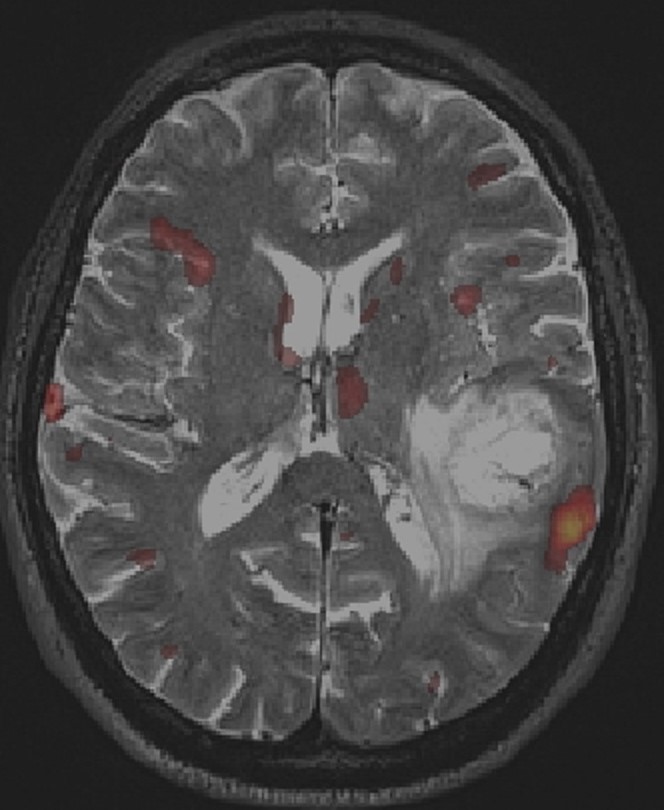
fMRI with BOLD imaging during object naming. The yellow/red BOLD signal displays significant activation in the left temporo-parietal region, in the expected anatomical area for language production, and in close proximity to the location of a glioblastoma
TREATMENT + CARE
Surgery
Surgery is the first step for the treatment of astrocytomas, as it provides two important benefits: First, it procures tumor tissue to establish a diagnosis. Secondly, it offers the possibility to remove as much tumor is safely possible to relieve mass effect, reduce swelling and facilitate response to adjuvant therapies, when indicated. The decision whether to perform a simple biopsy or a full resection depends on multiple factors, but particularly on the clinical and medical conditions of the patient, as well as the predicted extent of resectability of the tumor.
Important tools to maximize efficiency and safety of surgery are:
- Neuronavigation: it is, in essence, a GPS system for the brain, and allows the surgeon to visualize in real time on the MRI his/her localization within the patient’s brain. This significantly increases precision and minimizes the risk of injuring normal brain.
- Awake surgery: This technique is particularly useful to resect tumors located in speech areas, and also when close to primary motor cortex bilaterally. The patient is kept sedated but not intubated, so that he/she can speak and execute commands when asked to. In this way, the surgeon can continuously asses the patient’s functions while removing the tumor.
- Motor mapping during general anesthesia: The regions of the brain controlling movement can be also stimulated with an electrode even if the patient is asleep. A stimulator is used to apply currents directly to the brain cortex, and muscle responses are recorded.. Positive responses are interpreted as brain structures that should be spared from resection.
- Fluorescent dyes: Tumors, particularly those of higher grade, have the characteristic to avidly absorb certain dyes that are given IV to the patient just before surgery. In this way, the tumor tissue becomes colored by the specific dye, while the normal brain does not. This allows a much more precise definition of what should be resected and what should be left untouched. Among the most reliable dyes are 5-ALA, which colors the tumor violet, and Fluoresceine, which colors the tumor yellow, when visualized with appropriate lenses (see below)
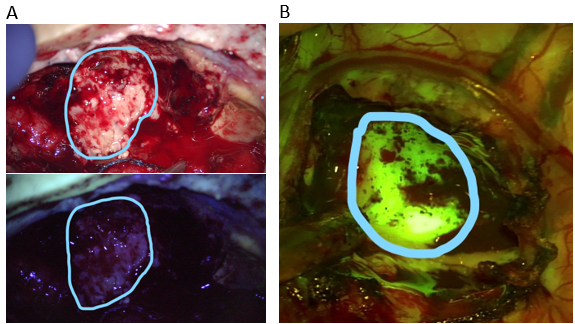
Intraoperative imaging of a recurrent high grade astrocytoma. Under white light (upper panel A) the tumor and necrotic portions are not clearly distinguishable. Under the blue-450 nm filter (lower panel A), the non-fluorescent darker portion represents radiation necrosis, while the bright magenta-pink fluorescence represents tumor tissue uptaking 5-ALA.
In another patient (panel B), the tumor is readily visible as a bright yellow-green hue after administration of fluoresceine and imaging with a 560 nm yellow filter
Established adjuvant therapies
- Steroids: Dexamethasone is the drug of choice to relieve symptoms due to the brain swelling that is often associated to the tumor. It is a very effective drug, which acts quickly and reliably. Unfortunately, it does not have any action against the tumor, and it is associated to significant side effects when used for periods longer than 2-3 weeks: weight gain, high blood sugars, hypertension, increased risk of infection, irritability.
- Chemotherapy with TMZ: Temozolomide (TMZ) is a drug which is taken by mouth and works by slightly modifying the DNA of tumor cells . This effect on the DNA triggers its breakage and consequent death of the cell, unless DNA repair mechanisms override the damage. TMZ is by now a well established first line treatment for every astrocytoma which is either grade 3 or 4 and is occasionally used also for grade 2 tumors (when they are not completely resected in surgery or if their genetic analysis is not favorable). It is usually taken daily for 5 days, followed by a rest period of 3 weeks, before starting another cycle. Side effects are rather bland, but it has been associated with anemia and fatigue. It is possible to predict the sensitivity of the astrocytoma to TMZ by measuring the activity of the MGMT gene, which is able to repair the damage induced by TMZ, and thus neutralizes the therapeutic effect of the drug.
- Radiotherapy: Radiation has been at the basis of treatment of astrocytomas for the past 50 years and it is extremely effective, at least for the first few months after treatment. Radiation, too, works by damaging DNA of the tumor cells, thus inducing their death. Under standard protocols, the treatment consists of small doses of radiation in the area of the tumor, 5 days a week for 6 weeks. Side effects are local hair loss (usually temporary), and fatigue. Long term side effects are necrosis of the brain around the treated region, and cognitive difficulties.
- Bevacizumab (Avastin): Bevacizumab is a drug that blocks the tumor’s ability to recruit blood vessels so that they can feed themselves and keep growing. Avastin has been approved by the Food and Drug Administration (FDA) in 2013 for its use in recurrent glioblastomas. It is very effective in reducing the swelling associated with the tumor and often helps improving symptoms. In this sense, it functions as a potent alternative to steroids. Unfortunately, differently from radiation and TMZ, it does not significantly prolong survival.
- Tumor treating electrical fields In 2011, the FDA approved the use of a special device applied to the scalp as a helmet, which produces low current electric fields which have been proved to delay tumor growth. They can be used both for recurrent glioblastomas, and, since 2015, also for newly diagnosed glioblastomas. For this device to be helpful, it needs to be worn at least 18 hours every day. Side effects are minimal and mainly consist of skin irritation.
- Antiseizure Drugs: Levetiracetam (Keppra) is the most widely used drug for this purpose. It is usually reserved only for patients that have already experienced at least one seizures.
Experimental Therapies
Astrocytomas, and in particular glioblastomas, are the target of intense research and every year several clinical trials are conducted to find new strategies which would improve survival. The most intense field of investigations are the following:
- Targeted therapies: Nowadays, the majority of treating centers perform detailed genetic analysis of any tumor tissue removed at surgery, in order to obtain patient-specific molecular signatures which can help establish the best drug for that tumor.
- Immunotherapy: A weakened immune surveillance is fundamental for the development and progression of astrocytomas. For this reason a large effort is currently underway to find strategies to boost the immune system against the tumor. This is being currently investigated with the use of antitumor vaccines, the use of genetically modified immune cells which are administered to the patient by intravenous infusion, or the use of drugs which stimulate the immune system activation.
- Virus therapy: A promising and ever expanding approach is the use of viruses which, once administered to patients (usually directly injected by the neurosurgeon into the tumor), can selectively infect and destroy tumor cells without harming the surrounding normal brain. Historically, the first virus to be investigated extensively has been Herpes Simplex (the cold sore virus). Recently, some encouraging results have been observed with the use of Poliovirus, even though the benefit was only observed in a small percentage of patients.
Prognosis
The major factors determining length of survival after a diagnosis of astrocytoma are the following:
- Tumor grade/histology
- Grade 1 tumors are largely cured (96% survival rate at 5 years), usually by surgery only.
- Grade 2 tumors: Overall median survival is 8 years. Presence of IDH1 mutation is associated with longer survival.
- Grade 3 tumors: Median survival is 3-5 years
- Grade 4 tumors: Median survival is 15 months.
- Extent of surgical resection: Although complete microscopic resection of grade 2-4 tumors is impossible due to their diffuse infiltration into the normal brain, macroscopically total resection is associated with significantly better survival. For GBM, an increase in survival is observed after at least 80% of tumor is resected and there is a stepwise improvement in prognosis as the extent of resection reaches the 95-100% of the contrast-enhancing component.
- Use of adjuvant radiotherapy and chemotherapy.
- Age: young age is associated with longer survival.
- Functional status: minimal symptoms/normal neurological function are associated with longer survival.
Sources
- Ostrom QT et al. Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol 2018; 4:1254-1262
- Ostrom QT et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol, 2019; 21, Supp 5: v1-v100
- Kyritsis AP et al. Inherited predisposition to glioma. Neuro Oncol, 2010; 12: 104-113
- Touat M et al. Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol, 2017; 28:1457-1472
- Villanueva-Meyer JE, et al. Clinical Brain Tumor Imaging. Neurosurgery. 2017; 81(3):397-415.
- Zakaria J, Prabhu VC. Cortical Mapping in the Resection of Malignant Cerebral Gliomas. In: De Vleeschouwer S, editor. Glioblastoma. Brisbane (AU): Codon Publications; 2017; Chapter 13.
- Barker FG II, Gutin PH. Surgical approaches to gliomas. In The Gliomas, Berger MS, Wilson CB, eds., WB Saunders Co. Philadelphia, PA, 1999.
- Byrne RW et al. Introduction: Advances in intraoperative brain mapping. Neurosurg Focus. 2018; 45(VideoSuppl2)
- Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016; 130(2):269-282
- Stummer W, Suero Molina E. Fluorescence Imaging/Agents in Tumor Resection. Neurosurg Clin N Am. 2017; 28(4):569-583.
Note from AANS
The AANS does not endorse any treatments, procedures, products or physicians referenced in these patient fact sheets. This information is provided as an educational service and is not intended to serve as medical advice. Anyone seeking specific neurosurgical advice or assistance should consult his or her neurosurgeon, or locate one in your area through the AANS’ Find a Board-certified Neurosurgeon online tool.
